台灣風險分析學會
Taiwan Society for Risk Analysis

2021年第4期
淺談美國環保署對環境內分泌干擾物和人類健康的觀點(下篇)
農業藥物毒物試驗所 呂水淵
Release: Apr 05, 2021
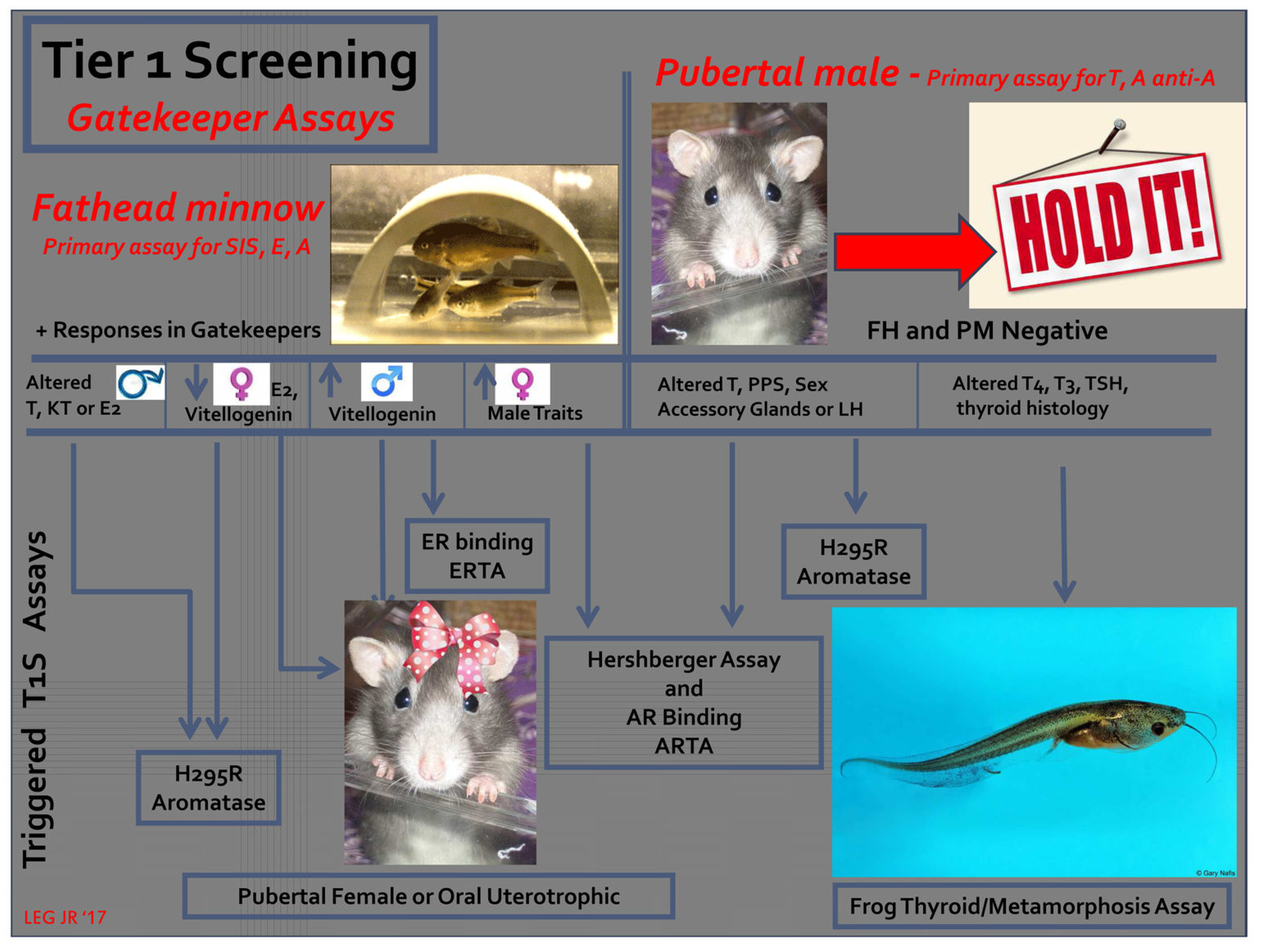
圖2.一種基於“證據權重”的文獻回顧,簡化11種EPA、EDSP 第1階篩選試驗的潛在方法,該文獻涉及測試指南方案中有關化學藥品的影響[4;18]。在這種方法中,最初進行了兩次看門(gatekeeper)分析,即黑頭呆魚短期生殖測試和青春期雄大鼠測試。如果這些均為陰性,則無需其他篩選。這取決於魚的檢測,以檢測雌性素和抑制類固醇生成的化學物質。在青春期雄性檢測中檢測到雄性素和抗雄性素以及會改變甲狀腺功能的化學物質。如果任一看門(gatekeeper)分析均為陽性(在無明顯毒性的情況下),則根據作用方式觸發額外的篩選[4; 16; 18]。(縮寫:T1S-EPA,EDSP一級篩選測定組合; AR-雄性素受體; ARTA(androgen receptor transcriptional antagonist assay)-雄性素受體轉錄拮抗劑測定; T (testosterone)-睾丸激素; PPS(preputial separation)-陰莖包皮分離; LH(luteinizing hormone)-黃體生成激素; T4(thyroxine)-甲狀腺素; T3(triiodothyronine)-三碘甲腺氨酸; TSH (thyroid stimulating hormone)–甲狀腺刺激激素; FH(fathead minnow reproduction test) –黑頭呆魚繁殖試驗; PM(pubertal male assay) –青春期雄性測定; E2 – 17β(estradiol-17β)雌二醇; KT(ketotestosterone) –酮睾酮; H295R(cell line used to assess steroidogenesis in vitro) –用於體外評估類固醇生成的細胞系[48]。
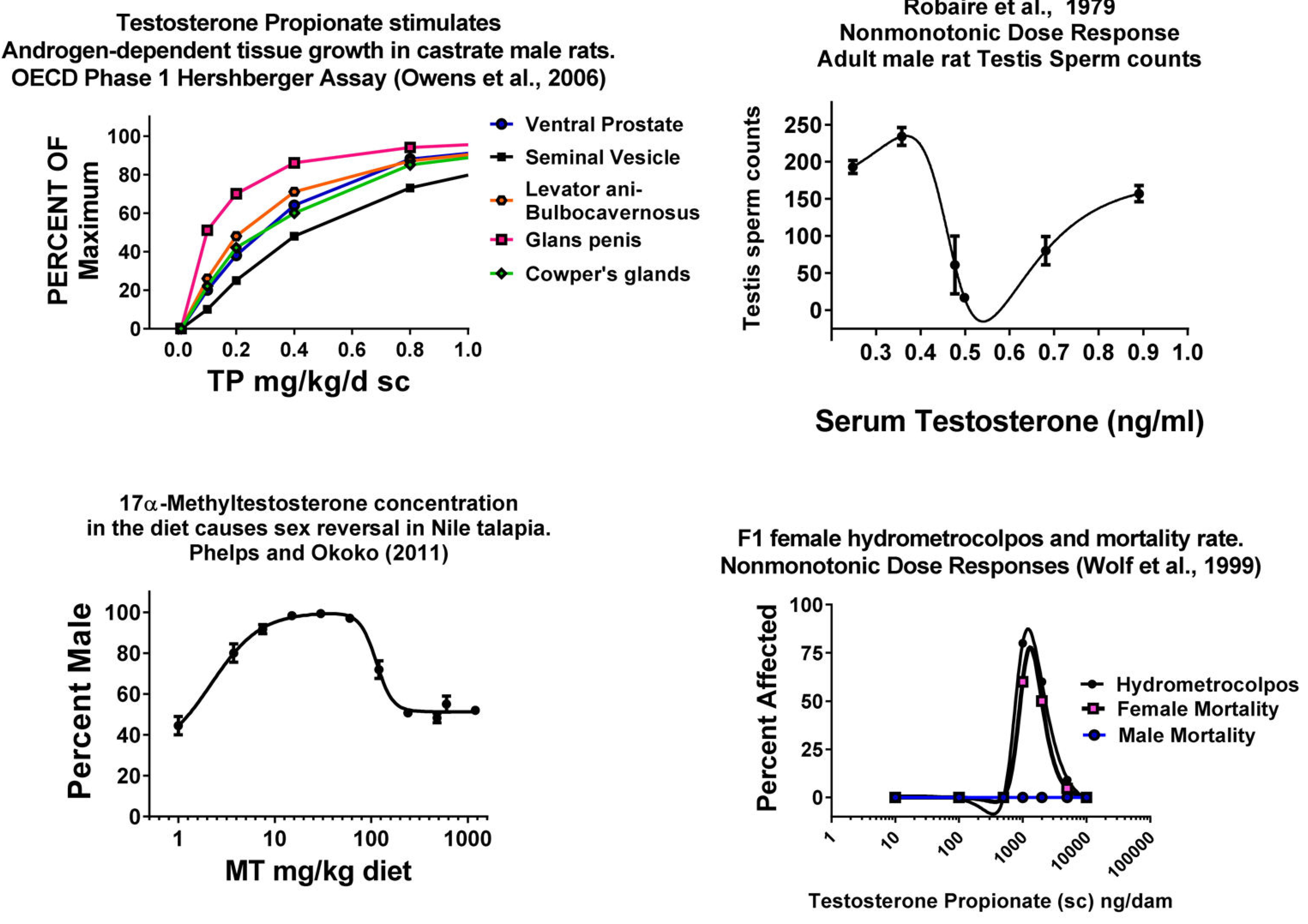
圖3.Owens等人(2006) [47],Robaire等人(1979) [30],Phelps和Okoko等人(2011) [33]和Wolf等人(2002)的脊椎動物生殖組織中的穩健體內NMDR和明顯的LNT劑量反應[32]。左上圖顯示了用丙酸睾丸酮(皮下注射)處理十天後稱重的五個雄性素依賴性組織的劑量相關反應。這些反應在低劑量範圍內似乎都是線性的。數據來自OECD在15個實驗室進行的實驗室間Hershberger分析驗證研究(n = 90,每個實驗室每劑量6隻大鼠)右上方的面板在長期使用睾丸激素(皮下植入)處理後,顯示出睾丸精子計數的非單調劑量反應(NMDR)。睾丸重量也顯示出相似的NMDR,而血清睾丸激素和LH則沒有。左下圖顯示了餵食中甲基睾丸激素對尼羅羅非魚性別比的影響的NMDR。右下面板。在子宮內施用丙酸睾丸激素(皮下注射)會引起NMDR,從而導致雌性大鼠後代的生殖道畸形和死亡。死亡率僅在雌性後代和青春期後才可見[48]。
參考文獻
1. Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray LE Jr. Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicology and applied pharmacology. 1994; 126:276–85. [PubMed: 8209380]
2. Kelce, WR., Monosson, E., Gray, LE. Biology of reproduction. SOC STUDY REPRODUCTION; 1603 MONROE ST MADISON, WI 53711–2021: 1994. IN-VITRO IN-VIVOEVIDENCE THAT VINCLOZOLIN (V) IS AN ENVIRONMENTAL ANTIANDROGEN; p. 102-102.
3. Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environmental toxicology and chemistry/SETAC. 2010; 29:730–41.
4. Ankley GT, Gray LE. Cross-species conservation of endocrine pathways: a critical analysis of tier 1 fish and rat screening assays with 12 model chemicals. Environmental toxicology and chemistry/ SETAC. 2013; 32:1084–7.
5. JECFA. Trenbolone acetate, Toxicological evaluation of certain veterinary drug residues in food. International Programme on Chemical Safety; Geneva, Switzerland: 1988.
6. Hotchkiss AK, Ankley GT, Wilson VS, Hartig PC, Durhan EJ, Jensen KM, Martinovi D, Gray LE. Of mice and men (and mosquitofish): antiandrogens and androgens in the environment. BioScience. 2008; 58:1037–1050.
7. Howdeshell KL, Hotchkiss AK, Gray LE Jr. Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment. International journal of hygiene and environmental health. 2016
8. Colborn, T., Clement, C. Chemically-Induced Alterations in Sexual and Functional Development: the Wildlife/Human Connection. Princeton Scientific Publishing Co., Inc; Princeton, NJ: 1992.
9. Hartig PC, Cardon MC, Blystone CR, Gray LE Jr, Wilson VS. High throughput adjustable 96-well plate assay for androgen receptor binding: a practical approach for EDC screening using the chimpanzee AR. Toxicology letters. 2008; 181:126–131.
10. Hartig PC, Bobseine KL, Britt BH, Cardon MC, Lambright CR, Wilson VS, Gray LE Jr. Development of two androgen receptor assays using adenoviral transduction of MMTV-luc reporter and/or hAR for endocrine screening. Toxicol Sci. 2002; 66:82–90.
11. Wilson VS, Bobseine K, Lambright CR, Gray LE Jr. A novel cell line, MDA-kb2, that stably expresses an androgen- and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicol Sci. 2002; 66:69–81.
12. Wilson VS, Bobseine K, Gray LE Jr. Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol Sci. 2004; 81:69–77.
13. Richard, AM., Truong, H., Wolf, M., Thillainadarajah, I. ToxCast Chemical Inventory: Data Management & Data Quality Considerations. 2014.
14. Gray LEJ. Quantification of the uncertainties in extrapolating from in vitro androgen receptor (AR) antagonism to key events in in vivo screening assays and adverse reproductive outcomes in F1 male rats. The Toxicologist. 2017:139.
15. Gray LE, Evans N, Wilson VS. Anti- and Androgenic Activities in MDA-kb2 Cells: A Comparison of Performance in 96 Well versus HTS Assays. The Toxicologist. 2015; 144(1):174.
16. Coady KK, Biever RC, Denslow ND, Gross M, Guiney PD, Holbech H, Karouna-Renier NK, Katsiadaki I, Krueger H, Levine SL, Maack G, Williams M, Wolf JC, Ankley GT. Current limitations and recommendations to improve testing for the environmental assessment of endocrine active substances. Integr Environ Assess Manag. 2016
17. Stump DG, O’Connor JC, Lewis JM, Marty MS. Key lessons from performance of the U.S. EPA Endocrine Disruptor Screening Program (EDSP) Tier 1 male and female pubertal assays. Birth defects research. 2014; 101:43–62.
18. Gray LE, Ankley GT. A Two-Tiered-Testing Decision Tree for Assays in the US EPA-EDSP Screening Battery: Using 15 Years of Experience to Improve Screening and Testing for Endocrine Active Chemicals. The Toxicologist. 2015; 144(1):183.
19. Juberg DR, Borghoff SJ, Becker RA, Casey W, Hartung T, Holsapple MP, Marty MS, Mihaich EM, Van Der Kraak G, Wade MG, Willett CE, Andersen ME, Borgert CJ, Coady KK, Dourson ML, Fowle JR 3rd, Gray LE, Lamb JC, Ortego LS, Schug TT, Toole CM, Zorrilla LM, Kroner OL, Patterson J, Rinckel LA, Jones BR. t4 workshop report--lessons learned, challenges, and opportunities: the U.S. Endocrine Disruptor Screening Program. Altex. 2014; 31:63–78.
20. Kang JJ, Chu SF, Wu ZZ, Chou SW, Tsai SJ, Chiu WT. Crisis management turns Taiwan’s plasticizer nightmare into progressive policy. J Formos Med Assoc. 2012; 111:409–11.
21. Chen PH, Chang KT, Lu YD. Polychlorinated biphenyls and polychlorinated dibenzofurans in the toxic rice-bran oil that caused PCB poisoning in Taichung. Bull Environ Contam Toxicol. 1981; 26:489–95.
22. Whorton D, Milby TH, Krauss RM, Stubbs HA. Testicular function in DBCP exposed pesticide workers. J Occup Med. 1979; 21:161–6.
23. Heaton SN, Bursian SJ, Giesy JP, Tillitt DE, Render JA, Jones PD, Verbrugge DA, Kubiak TJ, Aulerich RJ. Dietary exposure of mink to carp from Saginaw Bay, Michigan. 1. Effects on Gray reproduction and survival, and the potential risks to wild mink populations. Arch Environ Contam Toxicol. 1995; 28:334–43.
24. McKenzie RA, Freudigmann CL, Mawhinney H, Eaves LE, Green PE, Rees GJ. Dieldrin poisoning and botulism in Australian pelicans (Pelecanus conspicillatus). Australian veterinary journal. 1982; 58:148–52.
25. White RH, Cote I, Zeise L, Fox M, Dominici F, Burke TA, White PD, Hattis DB, Samet JM. State- of-the-science workshop report: issues and approaches in low-dose-response extrapolation for environmental health risk assessment. Environmental health perspectives. 2009; 117:283–7.
26. Conley JM, Lambright CS, Evans N, Cardon MC, Wilson VS, Gray LE Jr. Oral exposure to a mixture of 18 anti-androgens with varying mechanisms of action at concentrations below individual chemical effect levels produces reproductive tract malformations in the male rat. The Toxicologist. 2017
27. Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, Vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr Rev. 2012
28. Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Vom Saal FS. Endocrine-disrupting chemicals and public health protection: a statement of principles from the endocrine society. Endocrinology. 2012; 153:4097–4110.
29. Welshons WV, Nagel SC, Thayer KA, Judy BM, Vom Saal FS. Low-dose bioactivity of xenoestrogens in animals: fetal exposure to low doses of methoxychlor and other xenoestrogens increases adult prostate size in mice. Toxicology and industrial health. 1999; 15:12–25.
30. Robaire B, Ewing LL, Irby DC, Desjardins C. Interactions of testosterone and estradiol-17 beta on the reproductive tract of the male rat. Biology of reproduction. 1979; 21:455–63.
31. Ewing LL, Desjardins C, Irby DC, Robaire B. Synergistic interaction of testosterone and oestradiol inhibits spermatogenesis in rats. Nature. 1977; 269:409–11.
32. Wolf CJ, Hotchkiss A, Ostby JS, LeBlanc GA, Gray LE Jr. Effects of prenatal testosterone propionate on the sexual development of male and female rats: a dose-response study. Toxicol Sci. 2002; 65:71–86.
33. Phelps RP, Okoko M. A non-paradoxical dose response to 17α-methyltestosterone by Nile tilapia Oreochromis niloticus (L.): Effects on the sex ratio, growth and gonadal development. Aquaculture Research. 2011; 42:549–558.
34. Parrott JL, Bjerregaard P, Brugger KE, Gray LE Jr, Iguchi T, Kadlec SM, Weltje L, Wheeler JR. Uncertainties in biological responses that influence hazard and risk approaches to the regulation of endocrine active substances. Integr Environ Assess Manag. 2016
35. Ankley GT, Villeneuve DL. Temporal changes in biological responses and uncertainty in assessing risks of endocrine-disrupting chemicals: insights from intensive time-course studies with fish. Toxicol Sci. 2015; 144:259–75.
36. Matthiessen P, Ankley GT, Biever RC, Bjerregaard P, Borgert C, Brugger K, Blankinship A, Chambers J, Coady KK, Constantine L, Dang Z, Denslow ND, Dreier DA, Dungey S, Gray LE, Gross M, Guiney PD, Hecker M, Holbech H, Iguchi T, Kadlec S, Karouna-Renier NK, Katsiadaki I, Kawashima Y, Kloas W, Krueger H, Kumar A, Lagadic L, Leopold A, Levine SL, Maack G, Marty S, Meador J, Mihaich E, Odum J, Ortego L, Parrott J, Pickford D, Roberts M, Schaefers C, Schwarz T, Solomon K, Verslycke T, Weltje L, Wheeler JR, Williams M, Wolf JC, Yamazaki K. Recommended approaches to the scientific evaluation of ecotoxicological hazards and risks of endocrine-active substances. Integr Environ Assess Manag. 2017
37. Gray LE, Foster PM. Nonmonotonic Dose-Response Curves and Endocrine-Disrupting Chemicals: Fact or Falderal? The Toxicologist. 2013; 132(1):341.
38. Gray LE. Nonmonotonic Dose-Response Curves (NMDRCs) Are Common after Estrogen or Androgen Signaling Pathway Disruption—Fact or Falderal? The Toxicologist. 2013; 132(1):341.
39. Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environmental health perspectives. 2003; 111:994–1006.
40. Gray LE Jr, Ostby J, Monosson E, Kelce WR. Environmental antiandrogens: low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol Ind Health. 1999; 15:48–64. Find this article online.
41. Foster PM. Regulatory Forum opinion piece: New testing paradigms for reproductive and developmental toxicity--the NTP modified one generation study and OECD 443. Toxicologic pathology. 2014; 42:1165–7.
42. Gray L, Barlow N, Howdeshell K, Ostby J, Furr J, Gray C. Transgenerational effects of Di (2- ethylhexyl) phthalate in the male CRL:CD(SD) rat: added value of assessing multiple offspring per litter. Toxicological Sciences. 2009; 110:411–425.
43. Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE. Fifteen years after “Wingspread”--environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol Sci. 2008; 105:235–59.
44. Blystone CR, Kissling GE, Bishop JB, Chapin RE, Wolfe GW, Foster PM. Determination of the di- (2-ethylhexyl) phthalate NOAEL for reproductive development in the rat: importance of the retention of extra animals to adulthood. Toxicol Sci. 2010; 116:640–6.
45. Latendresse JR, Bucci TJ, Olson G, Mellick P, Weis CC, Thorn B, Newbold RR, Delclos KB. Genistein and ethinyl estradiol dietary exposure in multigenerational and chronic studies induce similar proliferative lesions in mammary gland of male Sprague-Dawley rats. Reproductive toxicology (Elmsford, NY. 2009
46. Greene RR, Burrill M, Ivy AC. Experimental intersexuality. The effect of antenatal androgens on sexual development of female rats. Am J Anat. 1939; 65:415–469.
47. Owens W, Zeiger E, Walker M, Ashby J, Onyon L, Gray LE Jr. The OECD program to validate the rat Hershberger bioassay to screen compounds for in vivo androgen and antiandrogen responses. Phase 1: use of a potent agonist and a potent antagonist to test the standardized protocol. Environmental health perspectives. 2006:1259–1265.
48. Gray LE Jr. Twenty-five years after” Wingspread”-Environmental endocrine disruptors (EDCs) and human health. Curr Opin Toxicol. 2017; 3: 40-47.